Carbon Element Overview
If you watched Star Trek, in one episode, the Nomad, the robot that referred to humans as carbon-based lifeforms, and for good reason. Because that’s what we are!
Virtually every organic compound on Earth contains carbon. Life as we know it would not exist without carbon. That’s because it has a unique ability to bond with itself and other elements fairly easily, due to its need to find more electrons to bond with.
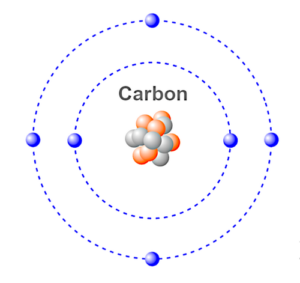
Carbon is the sixth element in the periodic table with the chemical symbol C and an atomic number of six. It has two electrons in its inner shell and four electrons in its outer shell (valence shell) as shown in the Bohr illustration above.
Because the carbon atom has a natural desire to fulfill its outer shell with eight electrons or saying it another way, it needs to fill up its outer energy level, it will constantly look to bond with other atoms to obtain four more electrons. Once bonded, the atom’s outer shell is fully stable. Carbon atoms can form bonds with other carbon atoms, but they can also form bonds with almost all other elements.
Carbon can exist in multiple different forms known as allotropes: graphite, diamond, and others. It’s also a non-metal, but one of the most important elements on earth. Carbon atoms have many uses, from making steel to fueling cars.
This article explores almost everything you wanted to know about carbon atoms and their various forms.
Types (Allotropes) of Carbon Molecules
Graphite

Graphite is an allotrope of carbon. It’s a black and soft mineral that is commonly found in nature in the form of pencils. Although graphite is often treated as a mineral, it’s more commonly considered a form of carbon. Graphite is very soft and can be easily compressed into a very thin sheet.
Graphite is made of layered sheets of carbon atoms that form stacks known as graphene. Each layer is made of carbon atoms arranged in a hexagonal pattern with strong covalent bonds. These layers are held together by weak intermolecular forces that are easily broken by heat. That’s why pencils can be erased by rubbing graphite and paper together!
The Diamond
The diamond is another allotrope of carbon. The only difference between the two is that diamonds are made of carbon atoms arranged in a cubic pattern. This makes diamonds a hard and rigid substance.
Diamonds are also made of graphene sheets that are held together by strong covalent bonds. These properties make this mineral extremely valuable, but they’re also highly limited in supply. That’s why they’re one of the most expensive materials on earth.
It’s estimated that only 0.1% of the carbon that enters the earth’s surface is converted into a diamond. This is large because diamonds are formed at very high pressures beneath the earth.
When carbon deposits are subjected to a combination of very high temperatures and pressure, they can change to diamonds. It may take a long time before the carbon is changed into a diamond, but it will change. It all depends upon the temperature and amount of pressure that is put on it. We can’t find a better demonstration than when Superman crushed coal (a product of carbon) simulating the creation of a diamond.
Carbon Bonds
The covalent bonds that can form carbon can result in many different types of molecules. Carbon can form thousands of bonds with other elements. This is why carbon has so many uses in the world.
Fullerenes
Fullerenes are carbon molecules that are composed of many rings of carbon. They were accidentally discovered in 1985 by two scientists who were studying carbon soot. The discovery was so exciting that the scientists won a Nobel Prize for their discovery!
C 60 – the most common carbon molecule – has 60 carbon atoms arranged in a spherical pattern. This sphere can be thought of as a football because the name “fullerene” comes from two English words: football and carbon.
C 60 is known as a buckyball and can be used as a tool for scientists. Yes, that’s what it’s called. Buckyballs are carbon atoms that are bonded to three other carbon atoms. Scientists can use buckyballs to study the structure of other molecules.
Why is There So Much Carbon in the World?
Carbon is the fourth most abundant element in the universe. Carbon is created in the interiors of stars and then released into the universe when those stars expire. It is present in the earth’s crust in the form of minerals and organic compounds. C 60, the largest buckyball, is only possible at a pressure of 100 gigapascals – the type of pressure that’s found inside giant planets. (A pascal is a unit of pressure. Gigapasclal is that unit of pressure x 1 billion).
Diatomic Carbon
Diatomic carbon is the simplest form of carbon. It contains two carbon atoms with one double bond between the atoms. A double bond is where an atom shares its valence electrons with two other atoms, in contrast to a covalent bond created by lighting and oxygen in the air, but it is usually destroyed by other compounds in the atmosphere.
This is important because diatomic carbon is a greenhouse gas. Carbon atoms are released into the atmosphere when plants are burned. These atoms are then oxidized by the other compounds in the air to create more diatomic carbon. Diatomic carbon is one of the most important greenhouse gases in the atmosphere. This is precisely why it was released in the first place!
Conclusion
Carbon is the element that forms the molecules for all known forms of life on earth. It’s the only element that can form molecules with a ratio of electrons to protons that’s necessary for biology.
Carbon is not a metal. Metals are largely defined by their electrical conductivity. Carbon is a non-metal and does not conduct an electrical current.

Carbon is also very common in the universe and can form multiple different types of bonds with other elements, so when Noman called humans carbon-based life forms, because of its abundance in the universe, maybe he met other carbon life forms in the galaxy we just don’t know about yet!








