 There are six metals in nature that are rarer than gold and possess their own unique properties. In this article, we will shed some light on these relatively unknown elements and their uses.
There are six metals in nature that are rarer than gold and possess their own unique properties. In this article, we will shed some light on these relatively unknown elements and their uses.
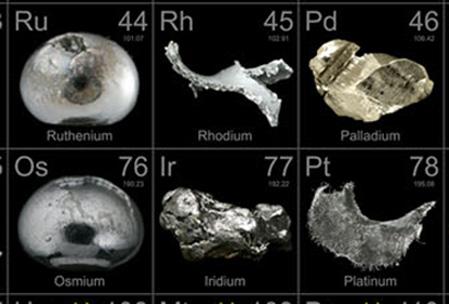
Ruthenium
In the 1840s, Russian chemist, Karl Ernst Claus, provided evidence for the existence of a new element in platinum ore. This new element was then named after the ancient name of Russia, Ruthenia.
Ruthenium has a silver-like sheen. It is a hard metal with a melting point between 2300 to 2450 degrees Celsius and a boiling point that ranges between 3900 to 4150 degrees Celsius. Ruthenium is a relatively non-reactive metal. It doesn’t dissolve in most acids and reacts only with those metals that have similar chemical properties. At room temperature, it doesn’t react to air, but higher temperatures can make it reactive to oxygen.
In nature, it is mostly found in platinum ores. Ruthenium is also obtained as a byproduct of nickel refining. This platinum metal is so rare that its abundance is only 0.0004 parts per million in nature.
Uses
Ruthenium is used in the production of different alloys due to its hardness and inertness to oxygen. Electrical contacts used to measure extreme temperatures usually contain ruthenium alloys.
Palladium
It resembles ruthenium in appearance, but has vastly different physical and chemical properties For instance, unlike ruthenium, it dissolves in aqua regia. Like other platinum group elements, palladium is mostly found in copper and nickel ore, however, small deposits of uncombined platinum have been found in Brazil. Palladium is 15 times rarer than platinum and is considered to be highly toxic and carcinogenic.
Uses
It is used in the making of an alloy — white gold — which is extensively used in jewelry making. Nowadays, palladium is being used in many electrical appliances as the component material of multi-layer ceramic capacitors.
Rhenium
Rhenium was discovered by a German team in the 1920s. It was the last discovered naturally occurring element. Chile, The United Kingdom, and Germany are major exporters of this rare metal. Rhenium is usually extracted from molybdenites and columbite ores.
Uses
Rhenium is used to make superalloys that are used to make parts of jet engines and gas turbine engines. They are also used in the making of temperature-controlling devices and heating elements.
Rhenium is also used as a catalyst to fracture the natural petroleum extracts into more useful products like gasoline, diesel.
Iridium
Iridium is another rare earth metal with a high density and a melting point. Its reactive tendencies are similar to that of gold. Iridium is also extracted during the process of nickel refining. Like other platinum family group members, it is very rare and used for very specific purposes.
Uses
Alloys made of iridium are used to make bearings used in compasses. Due to its high density and melting point, it is also used to make standard meter bars. It is also used as an electric contact in spark plugs due to its inertness and high melting point.
Rhodium
Rhodium is another rare metal from the same family of rare elements. In fact, it also resembles other metals of the group. Rhodium is highly conductive and is extremely resistant to corrosion.
Uses
Rhodium is used as a catalyst in the making of acetic acid, nitric acid and other hydrogenation reactions. One of the distinctive uses of rhodium is the part it plays in catalytic converters of cars. It is used to reduce the formation of nitric oxide in exhaust gases of the car.
Osmium
It is the densest of all the rare metals of the platinum family. It is a hard bluish metal with powerful properties as an oxidizing agent. It can be extracted from platinum-bearing ores in North America, South America and the Urals.
Uses
Due to its high density, it is used to make different instrument pivots and electrical contacts. An amorphous form of the metal can be used for staining on microscopic slides and detecting fingerprints.
The distinctive and unique uses of all these six rare metals tell us that while they belong to the same metal family, their properties go beyond the familial bond they share. Each individual metal has its own unique traits that distinguish it from the rest.
